
If I were to tell you that the next orbital is named “g”, would you be able to predict its height and width within an extended Periodic Table? Next, notice the width of each orbital block. A new block is introduced after every two rows. Notice first the height of each orbital block. By moving the s-orbital block to the right, we have further compromised macroscopic interrelationships in order to furnish ourselves with meaningful patterns and predictive power. It is important to realize that this reorganization in no way changes the atomic number ordering of Mendeleev, it simply rearranges it. The following idea (independently rediscovered by myself) was actually discovered in 1928 by Charles Janet. In fact, we can continue our journey towards the foundations for the periodic table with another reorganization. In effect, we allowed a certain amount of ambiguity with respect to the macroscopic in order to obtain greater clarity in the smaller scale. We have completely rearranged the Periodic Table into four separate rectangles, each of which represent different orbital types. They stand for sharp, principal, diffuse and fundamental.

These symbols originate from spectroscopy, and are regrettably more historical than meaningful.
Below we have delineated the four known groups into orbitals s, p, d, and f. It turns out that these rectangles represent orbitals, which stem from the principles of quantum mechanics. Remarkably, we discover that with our light manipulations, we are now able to group our Periodic Table into four separate rectangles. This symbolizes our other observation that chemists continue to discover higher-order elements. Finally, I have added an extended ellipsis at the lower right of the table. Allow me to further reposition helium – one of the exceptional elements mentioned above – alongside hydrogren. Let us assume that we are only interested in elements that are not ionized, then the order of an electron-based Periodic Table remains unchanged. The actual table looks like this:Ī lot less attractive, right? Well, before you leave dismayed by the chaos of nature, consider that the quantum mechanical approximation of orbitals can afford our table with an interpretation that can be considered beautiful. This last item means that the Periodic Table is, to a certain extent, oversimplified.
#Dynamic periodic table series#
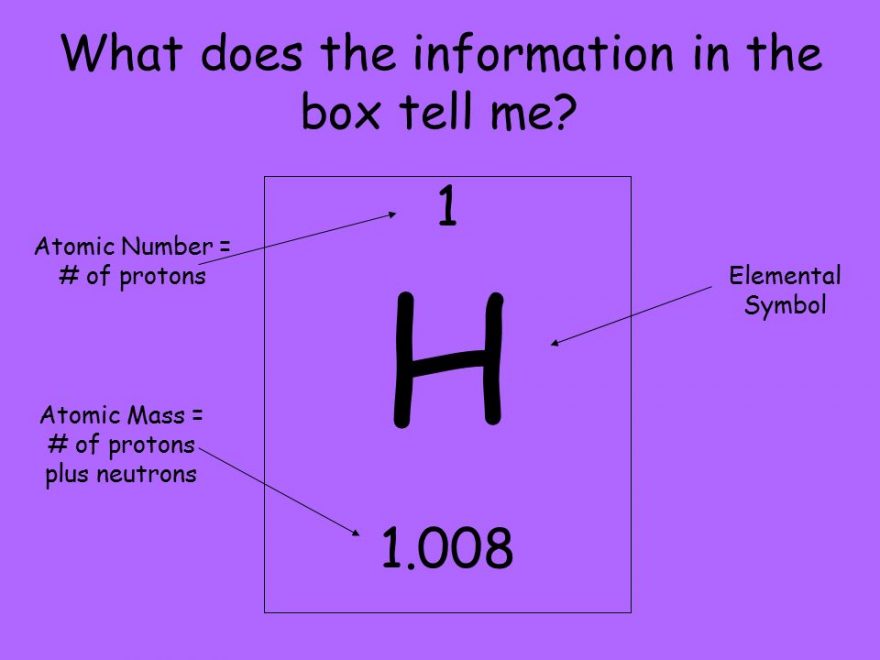
The Lanthanide and Actinide series are housed apart from the table (the two detached rows fit “inside” the yellow strip on the above table).Hydrogen and helium do not conform to their column’s traits as convincingly as other elements.Chemists have not discovered a last element new elements continue to be created.As we look closer, in fact, we find irregularities that demand an explanation: However, this brief synopsis of Mendeleev’s discovery is not comprehensive. The well-known elements of copper, silver and gold (located in the eighth column from the right, symbolized by Cu, Ag and Au respectively) illustrate this principle well: they are all strikingly less chemically reactive than nearby metals. Arranging these atoms into the table produces a startling result: electrons within the same column behave in strikingly similar ways. Indeed, all textbooks generally say the same thing: that these elements are arranged from left-to-right and then top-to-bottom in order of the amount of protons (the atomic number) in the nucleus. This illustration is a nice snapshot of the order that its creator, Dmitri Mendeleev, had found within the elements. Below I have constructed the Periodic Table as it appears in most textbooks. In this post, I would like to address the concepts of order and beauty within the Periodic Table. Content Summary: 600 words, 6 min reading time


 0 kommentar(er)
0 kommentar(er)
